- Solutions
- Industries
- KevSecure® Pharma Cloud for 300 Products
- KevSecure® for Pharma API
- Online Print & Inspect Systems for Cartons
- Online Print & Inspect Systems for Bottles
- Online Print & Inspect Systems for Labels
- Tamper Evident Online Print & Inspect Systems for Cartons
- Offline Print & Inspect Systems for Flat Cartons
- Offline Print & Inspect Systems for Folded Cartons
- Insights
- Support
- About
- Contact
INDIA DRUG BARCODING REQUIREMENTS
| REGULATORY AGENCY | India Ministry of Commerce and Industry, Department of Commerce, Directorate General of Foreign Trade (DGFT) |
| REGULATION NAME | Track and Trace System for Export of Pharmaceuticals and Drug Consignments |
| COMPLIANCE DATES | The date of implementation of Track and Trace system for export of drug formulations with respect to maintaining the Parent-Child relationship in packaging levels, and its uploading on Central Portal has been extended to 01.02.2025 SSI manufactured drugs. |
| APPLIES TO | Drug manufacturers who have manufacturing and/or packaging facilities within India, and who export drugs to any other countries. |
SECONDARY PACKAGING
(A carton containing one or more primary packs and includes a “mono carton” containing one primary pack)
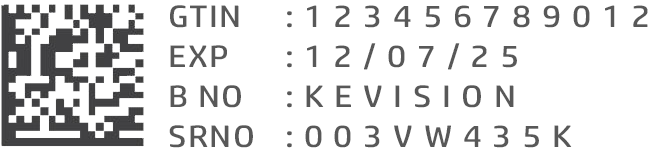
| BARCODE SYMBOLOGY | Linear, or 2D Datamatrix following GS1 standards |
| BARCODE CONTENTS |
|
| SERIAL NUMBER RANDOMIZATION | No |
| SERIAL NUMBER REUSE | Not Specified |
| HUMAN READABLE EXPIRY DATE FORMAT | YYMMDD |
| BARCODE DATA ENCODING | GS1 Standards |
| PRODUCT CODE NOTES | In the case of “mono cartons”, a manufacturer or exporter shall affix the bar code on mono cartons containing one primary pack on an optional basis until further notice |
| FREE SAMPLES MUST BE MARKED? | Not Specified |
TERTIARY PACKAGING
(A shipper containing one or more secondary packs)
| BARCODE SYMBOLOGY | GSl -128linear |  | |
| BARCODE CONTENTS | Product code (use of the GS1 GTIN is now optional), Lot Number,Expiration Date, GS1 SSCC (SSCC may appear in a separate barcode) | ||
AGGREGATION DATA CAPTURE
PARENT-CHILD MAPPING AGGREGATION CAPTURE? | Not Specified |
DATA EXCHANGE
| SEND UNIT DATA TO GOVERNMENT REPOSITORY? | Yes |
| SEND UNIT DATA TO THIRD-PARTY REPOSITORY? | As applicable |
| SEND UNIT DATA TO TRADING PARTNER? | As applicable |
| SEND AGGREGATION DATA TO GOVERNMENT REPOSITORY? | Yes |
| SEND AGGREGATION DATA TO THIRD-PARTY REPOSITORY? | As applicable |
| SEND AGGREGATION DATA TO TRADING PARTNER? | As applicable |
AUTHENTICATION
| WHO OFFERS DATA REPOSITORY FOR AUTHENTICATION? | Government (iVeda for now) |
| MANUFACTURERS MUST REGISTER SHIPMENTS IN REPOSITORY? | Yes (Exports) |
| DOWNSTREAM TRADING PARTNERS MUST AUTHENTICATE ON RECEIPT? | No (Future) |
| DOWNSTREAM TRADING PARTNERS MUST AUTHENTICATE ON SHIPMENT? | No |
GOVERNMENT REPORTING
| MANUFACTURER ACTIVITY REPORTED? | Yes |
| DOWNSTREAM TRADING PARTNER ACTIVITY REPORTED? | No |
INDIA DRUG BARCODING REQUIREMENTS
| REGULATORY AGENCY |
The Central Drugs Standard Control Organisation (CDSCO) |
| REGULATION NAME |
Drugs (Amendment) Rules, 2022 |
| COMPLIANCE DATES |
The new requirements will come into force on 1 January 2023. |
| APPLIES TO |
Every API manufactured or importers |
PRIMARY PACKAGING
(Packaging which is in direct physical contact with the active ingredient)| BARCODE SYMBOLOGY | As per the GS1 standards |
| BARCODE CONTENTS |
|
| SERIAL NUMBER RANDOMIZATION | No |
| SERIAL NUMBER REUSE |
Not Specified |
| HUMAN READABLE EXPIRY DATE FORMAT |
YYMMDD |
| BARCODE DATA ENCODING |
GS1 Standards |
| PRODUCT CODE NOTES |
QR code on its label at each level of packing that stored the data to facilitate tracking and tracing |
| FREE SAMPLES MUST BE MARKED? |
Not Specified |
SECONDARY PACKAGING
(A carton containing one or more primary packs and includes a “mono carton” containing one primary pack)| BARCODE SYMBOLOGY | As per the GS1 standards |
| BARCODE CONTENTS |
|
| SERIAL NUMBER RANDOMIZATION | No |
| SERIAL NUMBER REUSE |
Not Specified |
| HUMAN READABLE EXPIRY DATE FORMAT |
YYMMDD |
| BARCODE DATA ENCODING |
GS1 Standards |
| PRODUCT CODE NOTES |
QR code on its label at each level of packing that stored the data to facilitate tracking and tracing |
| FREE SAMPLES MUST BE MARKED? |
Not Specified |
TERTIARY PACKAGING
(A shipper containing one or more secondary packs)| BARCODE SYMBOLOGY | As per GS1 standard |  |
|
| BARCODE CONTENTS | GS1-128 (SSCC)
|
||
AGGREGATION DATA CAPTURE
|
PARENT-CHILD MAPPING AGGREGATION CAPTURE? |
Not Specified |
DATA EXCHANGE
| SEND UNIT DATA TO GOVERNMENT REPOSITORY? | Yes |
| SEND UNIT DATA TO THIRD-PARTY REPOSITORY? |
As applicable |
| SEND UNIT DATA TO TRADING PARTNER? |
As applicable |
| SEND AGGREGATION DATA TO GOVERNMENT REPOSITORY? | Yes |
| SEND AGGREGATION DATA TO THIRD-PARTY REPOSITORY? |
As applicable |
| SEND AGGREGATION DATA TO TRADING PARTNER? |
As applicable |
AUTHENTICATION
| WHO OFFERS DATA REPOSITORY FOR AUTHENTICATION? |
The Central Drugs Standard Control Organisation (CDSCO) |
| MANUFACTURERS MUST REGISTER SHIPMENTS IN REPOSITORY? |
Yes (Exports) |
| DOWNSTREAM TRADING PARTNERS MUST AUTHENTICATE ON RECEIPT? |
No (Future) |
| DOWNSTREAM TRADING PARTNERS MUST AUTHENTICATE ON SHIPMENT? | No |
GOVERNMENT REPORTING
| MANUFACTURER ACTIVITY REPORTED? | Yes |
| DOWNSTREAM TRADING PARTNER ACTIVITY REPORTED? |
No |
Source and Credit: This information is fully or partly based on the GS1 master document (all rights reserved). As GS1 does not control the content of the information, GS1 shall not be held liable, under any circumstance, if it differs from the master document.
DISCLAIMER
This information is being provided ‘As Is’ with no claims of suitability for a particular purpose. It represents just a possible interpretation of information available in the public domain and that interpretation is subject to change. This information does not constitute legal advice.
ABOUT KEVISION SYSTEMS
Kevision Systems, a part of the Kevin Group, is a leading solutions provider for complete end to end inspection of products & services for the inspection of pharmaceutical products like tablets, capsules and more. In addition to the inspection systems, Kevision’s Track & Trace Software & related equipment provide a fully integrated solution to the traceability of pharmaceutical products & Supply Chain Integrity.
Built on the cornerstone of strong quality focus, we deliver excellent solutions with very high quality & reliability.
Kevision Systems is dedicated and driven to provide the finest & the most optimum solutions for the Supply Chain Integrity as well as Optical Inspection Systems. We stand strong on the pillars of integrity, professionalism, innovation and expertise and have earned the credibility to work with any size of projects. We strive to excel in terms of quality and technology and be a preferred partner for our valued clientele.
For Further Information, please contact our Compliance Team at info@kevision.in