- Решения
- Smart Manufacturing
- Industries
- KevSecure® Pharma Cloud for 300 Products
- KevSecure® for Pharma API
- Online Print & Inspect Systems for Cartons
- Online Print & Inspect Systems for Bottles
- Online Print & Inspect Systems for Labels
- Tamper Evident Online Print & Inspect Systems for Cartons
- Offline Print & Inspect Systems for Flat Cartons
- Offline Print & Inspect Systems for Folded Cartons
- Insights
- About
- Support
- Contact
Track & Trace
KevSecure®
KevSecure, is a robust & stable Serialization Software that meets the various dynamic needs of Serialization as well as Regulatory requirements and is also very flexible to adapt to the various upcoming regulations.

Smart Manufacturing Solutions
Revolutionize Manufacturing
Kevision brings the future of manufacturing to your doorstep with our comprehensive Smart Manufacturing Solutions. By seamlessly integrating cutting-edge technologies, advanced automation, and data-driven intelligence, we empower businesses to achieve unprecedented levels of productivity, efficiency, and quality.
Inspection Solution
Panorama 360
Traditional systems leave room for error, but the Panorama 360 leaves nothing to chance. Powered by a sophisticated array of multiple cameras, our system eliminates blind spots by capturing the entire surface area of your product

Track & Trace
PrintSecure®
Kevision’s Serialization Solution for Cartons is a High-Speed printing and verification system for GS1 Supported 2d data matrix along with human-readable data. The system prints a unique serial number & other important batch data, on the cartons.

Track & Trace
AggreSecure®
Industrial Supply Chain is optimized by the use of shipping boxes or cartons & a Secure Supply Chain requires a parent-child relationship (aggregation) to be incorporated.

Level 1 Solutions
Pallet Aggregation
Transform your packaging and shipping processes with Kevision's Manual Pallet Aggregation System. This efficient solution simplifies the aggregation process, ensuring accurate tracking and traceability of pallets, while improving operational efficiency.

Global Provider of Serialization Solutions.

We are a leading Solutions provider for Serialization Systems, Track & Trace Systems and Camera based Inspection Systems for products in healthcare, food & beverage, tobacco & other industries
KEVSECURE SERIALIZATION SUITE
Level 3 Serialization & Aggregation Software

KevSecure
KevSecure, is a robust & stable Serialization Solution that meets the various dynamic needs of Serialization as well as Regulatory requirements and is also very flexible to adapt to the various upcoming regulations.
Learn MoreKevSecure - L3
KevSecure L3, is Kevision's State Of The Art solution for Track & Trace. Located at the Plant Level (L3), KevSecure L3’s powerful capabilities enable you to manage the entire serialization and aggregation processes at the plant level.
Learn MoreInspection Solutions
360° Dosage Inspection
True vision inspection with intelligence. Panorama 360 is equipped with AI-Based Vision Algorithms and AI-Based Teaching, allowing the system to learn product characteristics.
Learn more
Bar Code, Pharma & 2D Code Systems
VeriSecure Inspection System is designed for standard and customized Inspection of the raw material and finished products.
Learn more
Blister Inspection Systems
BliSecure is a high-resolution Vision (Camera) based verification system used for inspection of pharmaceuticals products on various forming materials.
Learn more
Tablet Inspection Systems
TabSecure - Tablet Inspection System is a high-resolution color camera-based inspection solution for tablets with rejection control.
Learn more
Pin Hole Detector
With the HAWK, Kevision has set a benchmark for in-process control of packaging of pharmaceutical products, especially blister strips.
Learn more



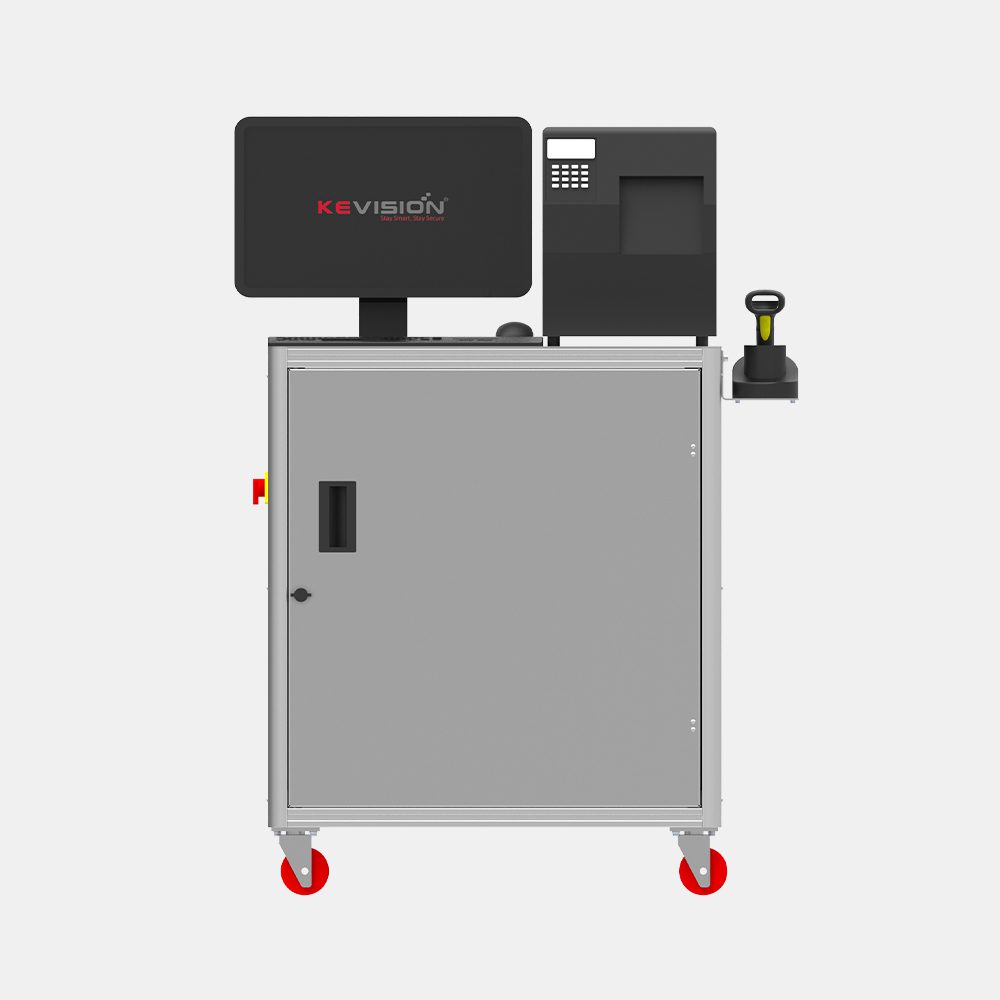
Print & Inspect Systems
PrintSecure
Kevision’s Serialization Solution for Cartons is a High-Speed printing and verification system for GS1 Supported 2d data matrix along with human-readable data.

Aggregation Systems
AggreSecure - Stellar
Kevision provides Semi-Automatic as well as Fully Automatic Aggregation Systems for aggregation between Cartons and Shipper or Bottles and Shipper.

Pallet Aggregation Systems
AggreSecure - Comet
Pallet Aggregation Station is connected with the Plant Server (L3) & provides functionality for Aggregation of Shippers on a Pallet. Kevision provides Automatic/Manual system for pallet aggregation
Testimonials of what
People are talking.

Google reviews
★★★★★ 5/5
Highly impressed with Kevision Systems' products! Their online printing and inspection solutions have greatly enhanced our pharmaceutical manufacturing processes.

Daniel
Working at Kevision Systems has been an absolute pleasure. The company fosters a culture of innovation and teamwork. Colleagues are supportive, and the leadership team is truly inspiring.

Amit
Kevision Systems' products have been a game-changer for our pharmaceutical company. Their track and trace solutions are indispensable in today's regulatory landscape.

Sudeep
They've helped us stay compliant with ever-changing regulations while boosting productivity.

Matthew
Joining Kevision Systems was a career-defining move. The work culture here is a perfect blend of professionalism and camaraderie.

Mira
The company values its employees and encourages professional growth. The collaborative spirit here is incredible, making it a great place to learn and thrive. Proud to be part of such a dynamic work culture!

Akash
Trusted by global brands. Join hundreds of customers around the globe.




























